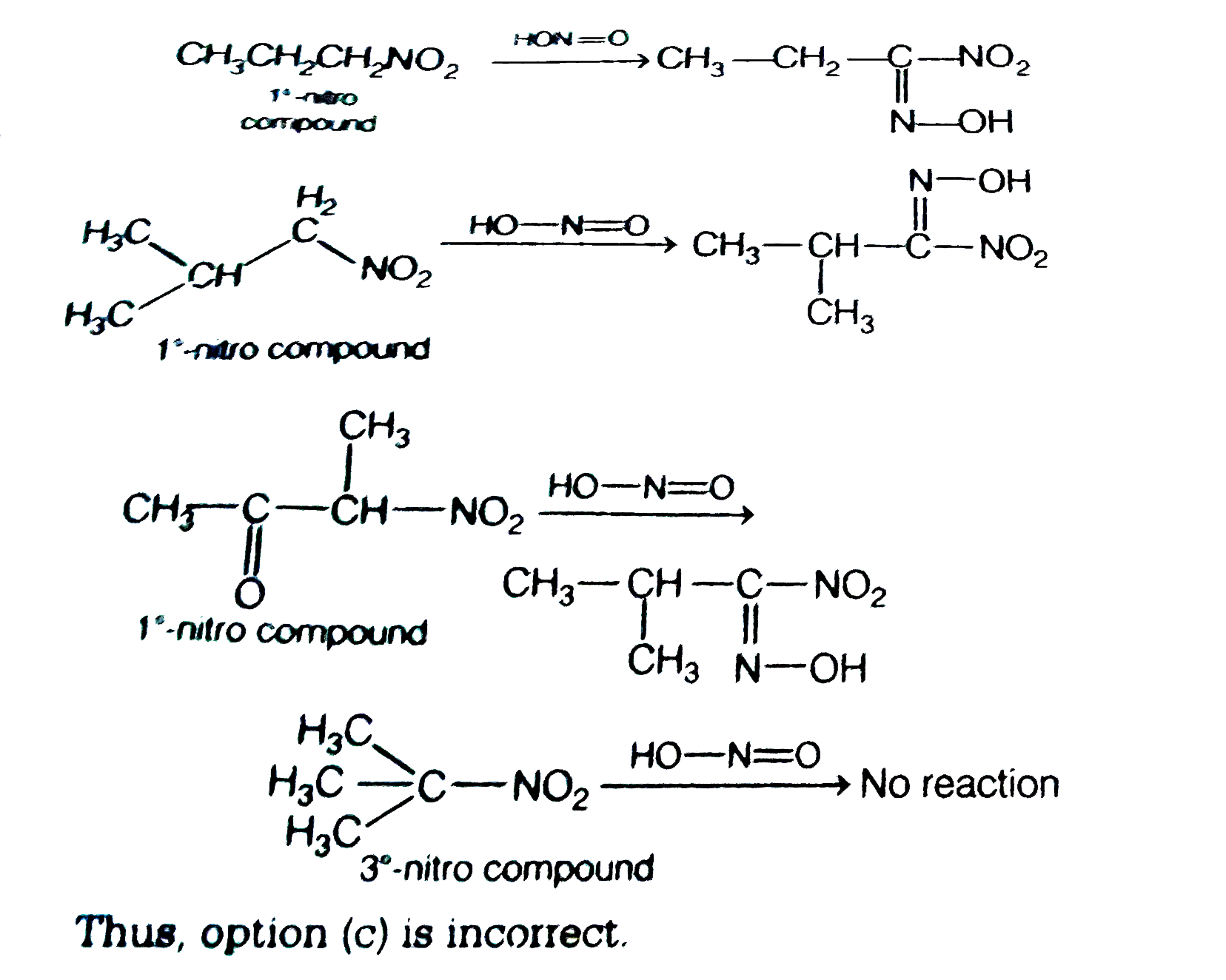Is Nitrous Acid Soluble . Nitrous acid is unstable and weakly acidic compound. Nitrous acid is a nitrogen oxoacid. In addition, it is a weak acid,. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. It is a conjugate acid of a nitrite. It is an oxoacid of nitrogen. It is soluble in water and has a pungent, suffocating odor. Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. It is obtained by acidification of nitrite. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a nitrite salt. It is used in the manufacture of explosives, in the production of. Nitrous acid is a monobasic acid, which means that each molecule of hno 2 releases one proton (h +) in solution. Nitrous acid is a strong acid that is a colorless, fuming liquid.
from www.doubtnut.com
In addition, it is a weak acid,. It is an oxoacid of nitrogen. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. It is used in the manufacture of explosives, in the production of. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a nitrite salt. Nitrous acid is a nitrogen oxoacid. It is obtained by acidification of nitrite. Nitrous acid is unstable and weakly acidic compound. It is a conjugate acid of a nitrite. Nitrous acid is a monobasic acid, which means that each molecule of hno 2 releases one proton (h +) in solution.
Which one of the following compounds does not react with nitrous acid
Is Nitrous Acid Soluble Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. It is used in the manufacture of explosives, in the production of. Nitrous acid is a monobasic acid, which means that each molecule of hno 2 releases one proton (h +) in solution. Nitrous acid is a strong acid that is a colorless, fuming liquid. It is a conjugate acid of a nitrite. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. It is soluble in water and has a pungent, suffocating odor. Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a nitrite salt. Nitrous acid is unstable and weakly acidic compound. In addition, it is a weak acid,. It is an oxoacid of nitrogen. It is obtained by acidification of nitrite. Nitrous acid is a nitrogen oxoacid.
From www.alamy.com
Molecule model salt hires stock photography and images Alamy Is Nitrous Acid Soluble Nitrous acid is a nitrogen oxoacid. In addition, it is a weak acid,. It is a conjugate acid of a nitrite. Nitrous acid is a strong acid that is a colorless, fuming liquid. It is an oxoacid of nitrogen. It is soluble in water and has a pungent, suffocating odor. Nitrous acid can be prepared by carefully adding a cold. Is Nitrous Acid Soluble.
From www.coursehero.com
[Solved] Which of the compounds is more soluble in an acidic solution Is Nitrous Acid Soluble It is a conjugate acid of a nitrite. Nitrous acid is a nitrogen oxoacid. It is an oxoacid of nitrogen. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. Nitrous acid is a strong acid that is a colorless, fuming liquid. In addition, it is a weak acid,. It. Is Nitrous Acid Soluble.
From www.numerade.com
⏩SOLVEDThe solubility of nitrous oxide is 0.12 g / 100 g water at Is Nitrous Acid Soluble Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. Nitrous acid is a monobasic acid, which means that each molecule of hno 2 releases one proton (h +) in solution. It is used in the manufacture of explosives, in the production of. Nitrous acid is unstable and weakly acidic compound. Nitrous acid, (hno2), an. Is Nitrous Acid Soluble.
From www.numerade.com
SOLVED 0.17M nitrous acid 0.12M sodium nitrite 0.25M ammonium Is Nitrous Acid Soluble Nitrous acid is unstable and weakly acidic compound. In addition, it is a weak acid,. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. It is obtained by acidification of nitrite. Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. Nitrous acid is. Is Nitrous Acid Soluble.
From mavink.com
Acid Solubility Chart Is Nitrous Acid Soluble It is obtained by acidification of nitrite. It is an oxoacid of nitrogen. Nitrous acid is unstable and weakly acidic compound. It is a conjugate acid of a nitrite. Nitrous acid is a monobasic acid, which means that each molecule of hno 2 releases one proton (h +) in solution. Nitrous acid can be prepared by carefully adding a cold. Is Nitrous Acid Soluble.
From webmis.highland.cc.il.us
Solubility and pH Is Nitrous Acid Soluble It is used in the manufacture of explosives, in the production of. It is soluble in water and has a pungent, suffocating odor. Nitrous acid is a nitrogen oxoacid. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a nitrite salt. Nitrous acid is a monobasic acid, which means that each. Is Nitrous Acid Soluble.
From www.dreamstime.com
Nitrous Acid Molecular Structure Isolated On White Stock Illustration Is Nitrous Acid Soluble Nitrous acid is a strong acid that is a colorless, fuming liquid. Nitrous acid is a nitrogen oxoacid. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. It is soluble in water and has a pungent, suffocating odor. Nitrous acid is unstable and weakly acidic compound. It is obtained. Is Nitrous Acid Soluble.
From rbk.bm
Solubility Rules Chart And Memorization Tips, 48 OFF Is Nitrous Acid Soluble Nitrous acid is a strong acid that is a colorless, fuming liquid. Nitrous acid is a nitrogen oxoacid. Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. It is used in the manufacture of explosives, in the production of. It is an oxoacid of nitrogen. Nitrous acid is unstable and weakly acidic compound. Nitrous. Is Nitrous Acid Soluble.
From www.bartleby.com
Answered Consider the following equilibrium for… bartleby Is Nitrous Acid Soluble It is soluble in water and has a pungent, suffocating odor. It is used in the manufacture of explosives, in the production of. It is obtained by acidification of nitrite. In addition, it is a weak acid,. It is a conjugate acid of a nitrite. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a. Is Nitrous Acid Soluble.
From www.numerade.com
SOLVEDWrite complete balanced halfreactions for (a) oxidation of Is Nitrous Acid Soluble Nitrous acid is a monobasic acid, which means that each molecule of hno 2 releases one proton (h +) in solution. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a. Is Nitrous Acid Soluble.
From thechemistrynotes.com
Benzoic Acid Definition, Preparation, Properties, Uses Is Nitrous Acid Soluble It is obtained by acidification of nitrite. Nitrous acid is a nitrogen oxoacid. It is an oxoacid of nitrogen. Nitrous acid is unstable and weakly acidic compound. Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. It is used in the manufacture of explosives, in the production of. Nitrous acid can be prepared by. Is Nitrous Acid Soluble.
From www.researchgate.net
(a) Crystal structure of Zn2(BDCNH2)2(DABCO) showing how 2D Is Nitrous Acid Soluble Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a nitrite salt. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. Nitrous acid is a. Is Nitrous Acid Soluble.
From www.animalia-life.club
Simple Hydrolysis Reaction Is Nitrous Acid Soluble Nitrous acid is a strong acid that is a colorless, fuming liquid. In addition, it is a weak acid,. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a nitrite salt. Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. It is a conjugate acid. Is Nitrous Acid Soluble.
From www.chemistrysteps.com
Arenediazonium Salts in EAS with Practice Problems Chemistry Steps Is Nitrous Acid Soluble It is a conjugate acid of a nitrite. It is obtained by acidification of nitrite. Nitrous acid is unstable and weakly acidic compound. Nitrous acid is a nitrogen oxoacid. It is soluble in water and has a pungent, suffocating odor. It is used in the manufacture of explosives, in the production of. Nitrous acid is a strong acid that is. Is Nitrous Acid Soluble.
From www.researchgate.net
Influence of the pH value on the solubility concentration of succinic Is Nitrous Acid Soluble Nitrous acid is unstable and weakly acidic compound. Nitrous acid (as sodium nitrite) is used as part of an intravenous mixture with sodium. In addition, it is a weak acid,. Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a nitrite salt. Nitrous acid, (hno2), an unstable, weakly acidic compound that. Is Nitrous Acid Soluble.
From www.alamy.com
Nitrous oxide molecule, illustration Stock Photo Alamy Is Nitrous Acid Soluble Nitrous acid can be prepared by carefully adding a cold and dilute solution of a strong acid to a nitrite salt. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form of cold, dilute solutions. It is used in the manufacture of explosives, in the production of. It is an oxoacid of nitrogen. It. Is Nitrous Acid Soluble.
From www.chegg.com
Solved A solution of nitrous acid has a pH of 2.45 . The Is Nitrous Acid Soluble It is an oxoacid of nitrogen. Nitrous acid is a nitrogen oxoacid. It is soluble in water and has a pungent, suffocating odor. Nitrous acid is a monobasic acid, which means that each molecule of hno 2 releases one proton (h +) in solution. Nitrous acid, (hno2), an unstable, weakly acidic compound that has been prepared only in the form. Is Nitrous Acid Soluble.
From www.animalia-life.club
Nitrous Acid Structure Is Nitrous Acid Soluble In addition, it is a weak acid,. It is an oxoacid of nitrogen. Nitrous acid is a strong acid that is a colorless, fuming liquid. It is a conjugate acid of a nitrite. It is used in the manufacture of explosives, in the production of. It is obtained by acidification of nitrite. Nitrous acid can be prepared by carefully adding. Is Nitrous Acid Soluble.